New European Cosmetic Regulation on Fragrance Allergens

In publication #010 of KosmetikOn Education we discuss the implementation of the new Allergens Regulation in the European Union's beauty industry, which aims to enhance transparency and inform consumers about allergenic ingredients in cosmetic products. This regulation introduces clear guidelines for labeling allergens in both rinse-off and leave-on products. It emphasizes primary and secondary prevention of fragrance allergies, introducing restrictions on allergen use and clear information for consumers. Providing an overview of the new Regulation explaining the rationale, the need for enhanced labeling, key changes introduced, and the practical steps manufacturers need to take to ensure compliance and enhance consumer safety and awareness.
New European Cosmetic Regulation on Fragrance Allergens
As the European Union continues to prioritize the safety and well-being of consumers, it has decided to go one step further to achieve greater transparency in the beauty industry. As of August 26 2023, the new Allergens Regulation enters into force, which will transform the way cosmetic products are labeled and presented to the public. This regulation introduces a series of clear and exhaustive guidelines for the labeling of allergens in cosmetic products, impacting both those that require rinsing and those that do not. This initiative seeks to inform consumers more accurately about the presence of allergenic ingredients in the products they use on a daily basis. Finally, In this article, we will cover the new labeling of allergens from its practical side and see how it will facilitate decision-making by consumers in their daily care routine.
Understanding the New European Cosmetic Regulation on Fragrance Allergens
Cosmetic products play a significant role in our daily lives, enhancing our personal care routines and boosting our confidence. However, the use of certain ingredients in cosmetics can sometimes lead to adverse reactions, particularly in individuals with sensitivities or allergies. In light of these concerns, the European Commission has taken a proactive step by introducing a new regulation aimed at enhancing the labelling of fragrance allergens in cosmetic products. This regulation, officially known as Commission Regulation (EU) 2023/1545, amends Regulation (EC) No 1223/2009 of the European Parliament and of the Council on cosmetic products. Let's delve into the key aspects of this new regulation and understand its implications.
The Need for Enhanced Labelling
Fragrance substances are widely utilized in cosmetic products, perfumes, and other household items due to their pleasant odors. Unfortunately, some individuals may develop contact allergies to certain fragrance allergens, leading to symptoms like eczema or allergic contact dermatitis upon exposure. Research suggests that approximately 1-9% of the population in the European Union is allergic to fragrance allergens. To address this issue, the new regulation emphasizes both primary prevention, aimed at reducing the risk of acquiring fragrance allergies, and secondary prevention, designed to protect sensitized individuals from allergy symptoms upon exposure. For primary prevention, restrictions on the use of fragrance allergens are implemented. However, for secondary prevention, it is crucial to provide consumers with clear information about the presence of specific fragrance allergens in cosmetic products, enabling those with sensitivities to avoid products containing allergenic substances.
Important Changes Introduced by the Regulation
In the past, only a limited number of fragrance allergens were required to be individually labelled on cosmetic products. However, the new regulation recognizes that more allergens have been identified as causing allergies in humans. To address this, the list of individually labelled allergens has been significantly expanded to include these newly identified substances.

Concentration Thresholds: The regulation introduces concentration thresholds for individually labelling fragrance allergens. When the concentration of a fragrance allergen exceeds 0.001% in leave-on products or 0.01% in rinse-off products, it must be individually labelled on the product packaging.
Summary: The regulation establishes concentration thresholds for the individually labelling of fragrance allergens. If the concentration of a fragrance allergen exceeds 0.001% in leave-on products or 0.01% in rinse-off products, it must be labelled on the product packaging.
Explanation: To provide clear guidance on when to label fragrance allergens, the regulation sets specific concentration thresholds. If the concentration of a particular allergen in a cosmetic product is above these thresholds, the allergen must be individually labelled on the packaging. This ensures that consumers are informed about the presence of potentially allergenic substances in the products they use. The minimum are 0.001% in leave-on products or 0.01% in rinse-off products, as in the previous regulation.
Equivalent Substances: Some fragrance substances can undergo chemical changes when exposed to air or certain biological processes, leading to the formation of known allergens. The regulation recognizes the potential risk posed by these transformed substances and treats them as equivalent to fragrance allergens. This means they must adhere to the same restrictions and labelling rules as other fragrance allergens.
Alignment and Clarity: To enhance the clarity and simplicity of product labelling, the regulation updates the way fragrance allergens are listed in cosmetic product ingredients. It aligns names with a common glossary, which makes it easier for consumers to understand the ingredients. Additionally, similar substances are grouped together for efficient labelling, and in cases of multiple names for a substance, a specific name is designated to streamline the labelling process.
Transition Period: Manufacturers and distributors of cosmetic products, are given time to adjust their products to meet the new labelling requirements. This transition period allows for changes in formulations and packaging. For products that were already on the market before the new requirements came into effect, a period is provided for their withdrawal or adjustment. The duration of the transition period varies depending on whether it concerns existing restrictions or new labelling provisions.
Practical Implications of the New European Cosmetic Regulation
The new European Cosmetic Regulation (EU) 2023/1545 has significant practical implications for both new and existing cosmetic products in the market. It introduces stricter requirements for the labelling of fragrance allergens, aiming to enhance consumer safety and awareness. Let's explore the practical steps that need to be conducted for both new and existing products to comply with the regulation.
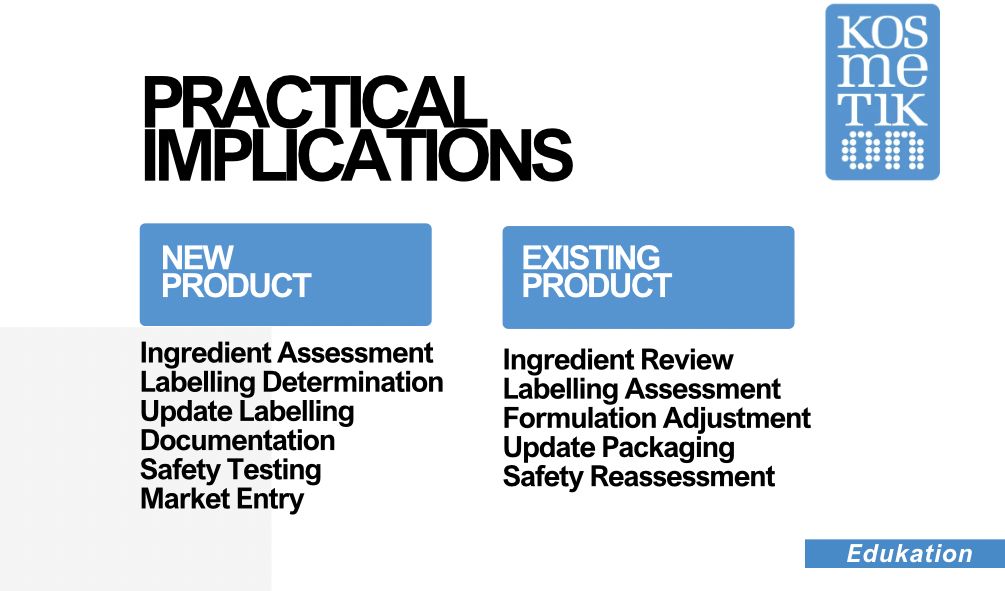
For a New Product:
Ingredient Assessment: Manufacturers must assess the ingredients in the new cosmetic product to identify any fragrance allergens present. This assessment involves determining the concentration of each allergen in the formulation. New allergens certifications based on the new regulation have to be prepared by the fragrance producers.
Labelling Determination: Based on the concentration of fragrance allergens, manufacturers must decide whether each allergen needs to be individually labelled on the product packaging.
Update Labelling: If the concentration of any fragrance allergen exceeds the specified thresholds, and the company do ot want to have them listed, it should be considered adjusting the formulation to reduce the allergen content. Additionally, the product packaging to include the individually labelled allergens must be updated. Perhaps it could be good news to be allowed to use a QR code for giving access to this huge ingredient list that will be now mandatory because the space in labels is limited.
Documentation: All assessments, labelling decisions, and formulation changes should be thoroughly documented for compliance purposes.
Safety Testing: Manufacturers should conduct appropriate safety tests to ensure that the formulation meets the required safety standards, even with reduced allergen content.
Market Entry: Once the formulation, labelling, and safety testing are completed, the new cosmetic product can be introduced to the market, bearing the necessary labelling information.
For an Existing Product:
Ingredient Review: Manufacturers of existing cosmetic products need to review their formulations to identify the fragrance allergens present and their respective concentrations.
Labelling Assessment: Determine whether the fragrance allergen concentrations exceed the specified thresholds. If they do, the allergens should be individually labelled on the product packaging.
Formulation Adjustment (if necessary): If allergen concentrations are above the thresholds, manufacturers may need to adjust the formulation to reduce the allergen content.
Update Packaging: Revise the packaging of the existing product to include the individual labelling of fragrance allergens that exceed the thresholds.
Safety Reassessment: Perform safety tests on the adjusted formulation to ensure that it complies with safety standards, taking into account the changes made. If there have been no changes in the formulation, previous tests are valid.
Transition Period: There is a transition period of 3 years for new products and 5 years for existing products on the market to implement the necessary changes.
In Summary:
The new European Cosmetic Regulation introduces more stringent labelling requirements for fragrance allergens in cosmetic products. Both new and existing products must undergo thorough assessments, potential formulation adjustments, labelling updates, safety testing, and compliance documentation. Manufacturers and economic operators must work diligently to ensure that their products meet the new regulation's standards, enhancing consumer safety and awareness in the process.
Kosmetikon will help you in all this process:
- The new allergens will be ready on the ingredients.
- The documentation will be asked to the manufacturers.
- A system for its implementation will be prepared.
- The formulas will be identified and its new Ingredient list prepared.
References
- Commission Regulation (EU) 2023/1545 of July 26 amending Regulation (EC) No. 1223/2009 of the European Parliament and of the Council on the labelling of fragrance allergens in cosmetic products. https://eur-lex.europa.eu/eli/reg/2023/1545/oj.