World cosmetic legislation in 2022
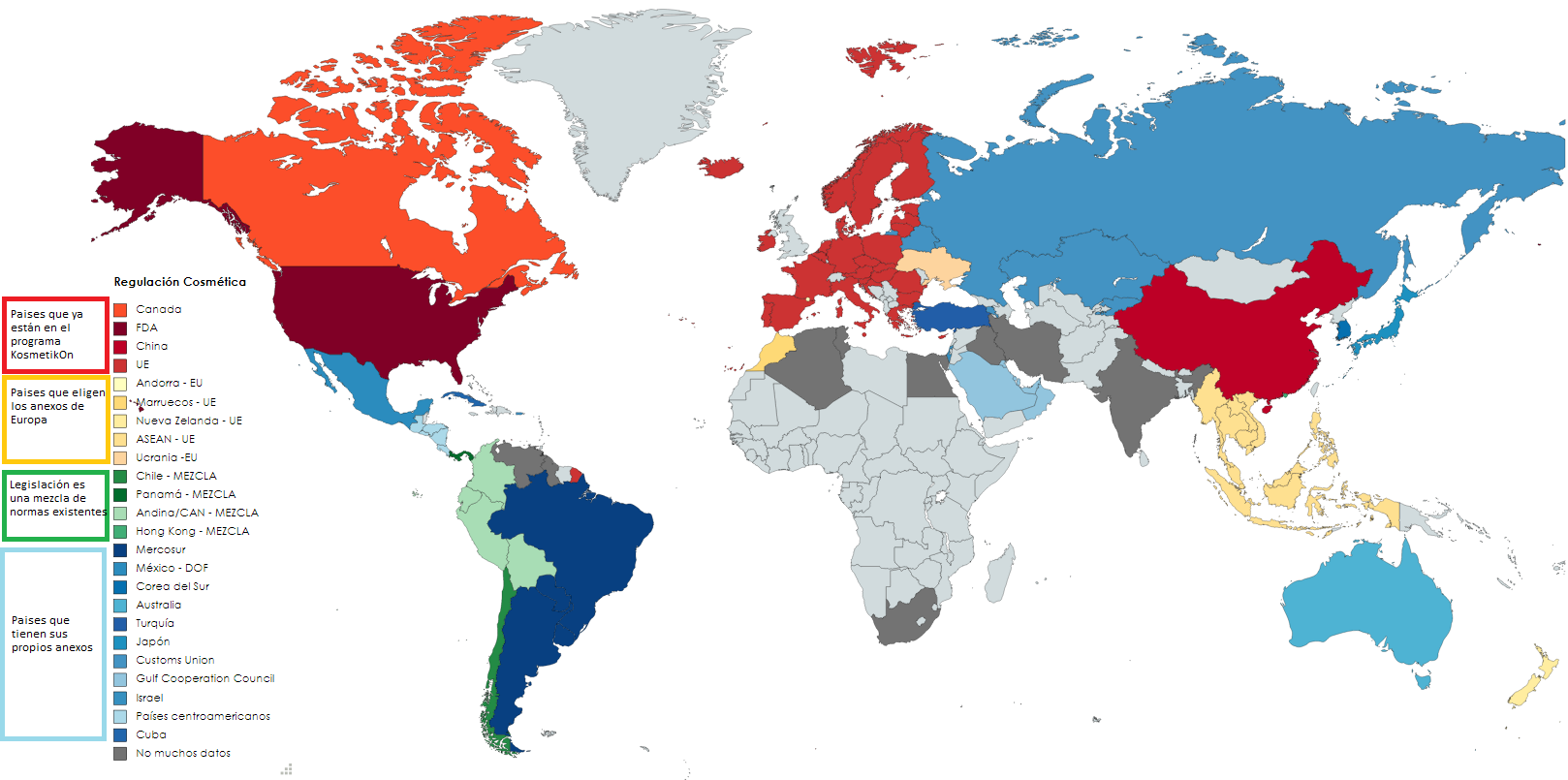
Designing a global cosmetic is difficult. There are various cosmetic laws that the product must comply with so that it can be sold in each region. It becomes even more complicated if the same formula is to be marketed in different markets. We summarise the information we have collected internally to add to our program.
European regulation
It includes the countries of the EU [1], made up of twenty-seven member countries: Germany, Austria, Belgium, Bulgaria, Cyprus, the Czech Republic, Croatia, Denmark, Slovakia, Slovenia, Spain, Estonia, Finland, France, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, the Netherlands, Poland, Portugal, Romania and Sweden. They also follow this legislation; Andorra, ASEAN [2], Brunei Darussalam, Cambodia, Indonesia, Laos, Malaysia, Myanmar, Philippines, Singapore, Thailand and Vietnam), Morocco, New Zealand, Ukraine.
These countries follow European legislation, mainly for authorised and regulated ingredients, following the legal requirements and restrictions that are published in annexes II (Substances prohibited in cosmetic products), III (Substances that cosmetic products cannot contain except if they comply with specified restrictions), IV (Allowed Colorants), V (Allowed Preservatives) and VI (Allowed UV Filters) of the legislation. Small differences must be taken into account, especially with regard to the language of the label, the language of the label must be that of the country where the product is marketed. Apart from other more specific legislative points.
Other Legislation
Australia [3], There is no single list of banned or restricted chemicals that you can view or download. Prohibitions and restrictions on chemicals and ingredients in consumer products, including cosmetics, are regulated by each state and territory authority. Every cosmetic product imported or manufactured must comply with any regulatory obligation attached to the import or manufacture of each chemical ingredient.
Canada [4], Apart from the ingredients with trivial names that must contain their designation in French and according to the European and/or United States nomenclature, the rest of the ingredients must follow the nomenclature of the ICI Dictionary [5]. The Canadian regulation forms a HOTLIST that is made up of 2 lists: prohibited ingredients and Restricted ingredients.
China [6], The documentation appears in Chinese, a regulatory publication from 2015 is currently in force, but a new one from the current year is about to be implemented. The summary of this document; Chapter 1 provides an overview, including scope, terminology and interpretation, and general requirements for cosmetic safety; Chapter 2 contains the requirements for prohibited and restricted components of cosmetics, including 1388 prohibited and 47 restricted components of cosmetics; Chapter 3 contains the requirements for permitted cosmetic components, including 51 permitted preservatives, 27 permitted sunscreens, 157 permitted colorants, and 75 permitted hair dyes. The other chapters describe various methods and permitted tests.
South Korea [7], Its regulations specify 1,030 prohibited ingredients; restricted use ingredients, 59 ingredients such as preservatives, 30 ingredients such as sunscreens and 75 ingredients with other restrictions and colorants, specifying type, quantity of colorant allowed in cosmetics.
Cuba [8], The qualitative composition according to its percentage participation in the INCI or CTFA nomenclature, according to the Cuban Standard INC 698; In the list of ingredients, the most common chemical name will be used in accordance with the International Nomenclature of Cosmetic Ingredients NIIC (INCI in English). For the language to be used, see chapter 7 of this standard.
Customs Union [9], Union of the countries Russia, Kazakhstan, Belarus, Armenia and Kyrgyzstan are part of this Customs Union that has adopted a common legislation on cosmetics that came into force on July 1, 2012. There is a list of prohibited substances, restricted substances, dyes and permitted pigments, preservatives and UV filters that must be met by cosmetic product formulas, similar to the European Union regulation, but with some differences.
FDA [10], In the US there is a limitation on the ingredients, there is a list of prohibited ingredients, and they cannot have drug activity, there are limitations on UVA filters and colorants. Specifically. California [11] presents several specific and more restrictive regulations for the cosmetics that are marketed there.
Gulf Cooperation Council (GCC) [12], is a union of the following countries: Saudi Arabia, Kuwait, United Arab Emirates, Qatar, Bahrain, Oman. The legislation is organised in the same way as the EU Regulation when it comes to restricted and prohibited substances. Annex II lists prohibited substances, Annex III lists restricted substances, and Annexes IV to VI list permitted colorants, preservatives, and UV filters; the list of such ingredients is also very similar to that of the EU.
Israel [13], Approved Ingredients, Banned Substances, Restricted Substances, Allowed Colorants/Pigments, Preservatives and UV Filters used in the formulas are quite similar to the EU regulations. However, the lists are not exactly the same and not all ingredients approved for use in the EU are approved on the Israeli market.
Mercosur [14], Cosmetic regulations in Mercosur (Argentina, Brazil, Uruguay and Paraguay) are 100% harmonised through Mercosur regulations (GMC [15]). There are prohibited ingredients, with restrictions, list of allowed UV filters.
Mexico [16], Products intended to be marketed in the national market must bear a label with the information established in this standard in Spanish. For the nomenclature of the ingredients, any of those established in the Agreements, or the most usual chemical name or the name as it appears in the International Nomenclature of Cosmetic Ingredients (INCI), may be used at the manufacturer's choice.
Central American countries [17], Guatemala, Honduras, Nicaragua, Costa Rica and El Salvador. Reference is made to Annex II, Prohibited Substances, Annex III, Restricted Substances. It resembles European legislation and they refer to it.
Turkey [18], The Turkish Government has been adapting its skin care cosmetic regulation to that of the European Union, as a consequence of the process of accession to the European Union. Currently, a draft that modifies the cosmetic regulations is under analysis.
UK [19], Since Brexit, the United Kingdom legislates to implement its own legislation. It is based on EU legislation and the successive legislative publications configure the obligations of the products marketed in England, Wales, Scotland and Northern Ireland.
Mix of different Legislations
Andean/CAN [20], Region formed by the countries Colombia, Bolivia, Peru and Ecuador. Their specific legislation is a mixed compliance with FDA and EU legislation. As an example, the list of ingredients must be presented in the international or generic INCI nomenclature (International Nomenclature of Cosmetic Ingredients).
Chile [21], For cosmetic products marketed in Chile, the official lists of the European Union, the standards of the Food and Drug Administration of the United States of America, and the recommendations of recognised national or international technical organisations will be used as reference. The lists of ingredients must be published annually in the Official Gazette, for purposes of adequate publicity.
Hong Kong [22], traded products may comply with international safety standards (such as the standards of mainland China, the United States, the European Union, Australia, or Japan) and will be deemed to comply with the requirements of its Ordinance.
Panama [23], Recognises the following references on ingredients that may or may not be incorporated into cosmetics and their corresponding restrictions or conditions of use: Personal Care Council (PCPC), The European Commission Cosmetic Ingredient Database (CosIng), European Union Directives and Central American Technical Regulations of Cosmetics.
References
- EUR-Lex site.Cosmetic Sector available at https://ec.europa.eu/growth/sectors/cosmetics_en (July. 2022)
- Australian Government Department of Health and Aged Care. Cosmetics and soap available at https://www.industrialchemicals.gov.au/cosmetics-and-soap (July. 2022)
- Government Canada. Guide to Cosmetic Ingredient Labelling available at https://www.canada.ca/en/health-canada/services/consumer-product-safety/reports-publications/industry-professionals/guide-cosmetic-ingredient-labelling.html (July. 2022)
- Personal Care Products Council. International Cosmetic Ingredient Dictionary and Handbook available at http://webdictionary.personalcarecouncil.org/ctfa-static/online/FrontMatter_Vol1%20Edited%20for%20Websites.pdf (July. 2022)
- National Institues for Food and Drug Control. Safety and Technical Standards for Cosmetics available at https://www.nifdc.org.cn/nifdc/xxgk/ggtzh/tongzhi/2022033116124546136.html (July. 2022) 6.Ministry of Food and Drug Safety. Cosmetics Act available at https://www.mfds.go.kr/eng/brd/m_28/view.do?seq=69983&srchFr=&srchTo=&srchWord=&srchTp=&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&page=3 (July. 2022)
- Instituto Nacional de Higiene, Epidemiologia y Microbiologia. Registro Sanitario de alimentos, cosméticos, juguetes y otros productos de interés sanitario: Regulacioines e indacadores available at
https://instituciones.sld.cu/inhem/files/2017/12/Manual-Registro-Sanitario-2017.pdf (July. 2022) - Customs Union Commission. Decision of 23 September 2011 N 7992: The safety of perfumery and cosmetics available at http://www.rustandard.com/images/CU_TR/TR_TS_009-2011.pdf (July. 2022)
- U.S Food And Drug Administration. Cosmetics Laws & Regulations. https://www.fda.gov/cosmetics/cosmetics-guidance-regulation/cosmetics-laws-regulations available at (July. 2022)
- California Legislative information. Health and Safety Code CHAPTER 14. Cosmetic Safety available at https://leginfo.legislature.ca.gov/faces/billTextClient.xhtml?bill_id=201920200AB2762 (July. 2022)
- Secretariat General of the Gulf Cooperation Council available at https://www.gcc-sg.org/en-us/Pages/default.aspx (July. 2022)
- Government services and information for Israel available at https://www.gov.il/en/search?query=cosmetics%20 (July. 2022)
- The Southern Common Market MERCOSUR available at https://www.mercosur.int/quienes-somos/en-pocas-palabras/ (July. 2022)
- Ministerio de Justicia y Derechos Humanos. Reglamento Técnico Mercosur sobre Rotulado para Productos de Higiene Personal, Cosméticos y Perfumes. Derogación de las Resoluciones GMC 36/99 Y 36/04 available at http://www.saij.gob.ar/48-internacional-reglamento-tecnico-mercosur-sobre-rotulado-para-productos-higiene-personal-cosmeticos-perfumes-derogacion-resoluciones-gmc-36-99-36-04-rmr2021000048-2022-03-02/123456789-0abc-de8-4000-01202rserced?q=%28organismo%3ACMC%29%20OR%20%28organismo%3AGMC%29&o=9&f=Total%7CFecha%7CEstado%20de%20Vigencia%5B5%2C1%5D%7CTema%5B5%2C1%5D%7COrganismo%5B5%2C1%5D%7CAutor%5B5%2C1%5D%7CJurisdicci%F3n%5B5%2C1%5D%7CTribunal%5B5%2C1%5D%7CPublicaci%F3n%5B5%2C1%5D%7CColecci%F3n%20tem%E1tica%5B5%2C1%5D%7CTipo%20de%20Documento&t=3222 (July. 2022)
- Diario Oficial de la Federación. Norma Oficial Mexicana NOM-141-SSA1/SCFI-2012, Etiquetado para productos cosméticos preenvasados available at http://himfg.com.mx/descargas/documentos/transparencia/pot/fraccion_xiv/382norma57.pdf (July. 2022)
- Government of Salvador Unidad de Registro de Cosméticos e Higiénicos available at https://www.medicamentos.gob.sv/index.php/es/servicios-m/descargables/unidad-de-registro-de-cosmeticos-e-higienicos (July. 2022)
- Presidency of the Republic of Turkey. Regulatory Information system available at https://www.mevzuat.gov.tr/mevzuat?MevzuatNo=8157&MevzuatTur=7&MevzuatTertip=5
- UK government. The Product Safety and Metrology etc. (Amendment etc.) (EU Exit) Regulations 2019, SCHEDULE 34 available at https://www.legislation.gov.uk/uksi/2019/696/schedule/34/made. (July. 2022)
- La Comision de la Comunidad Andina. Decision 777, Armonización de legislaciones en materia de productos cosméticos available at https://www.tribunalandino.org.ec/decisiones/normativa/DEC777.pdf (July. 2022)
- Biblioteca del congreso Nacional de Chile. Aprueba reglamento del sistema nacional de control de cosmeticos available at https://www.bcn.cl/leychile/navegar?idNorma=211455 (July. 2022)
- The government of Hong Kong Special Administrative region. Regulation of ingredients and labelling of personal care products and cosmetics available at https://www.info.gov.hk/gia/general/201806/27/P2018062700331.htm (July. 2022)
- Gobierno de la República de Panamá. Decreto Ejecutivo N° 40 que regula la ley 1 de 10 de enero de 2001, sobre medicamentos y otros productos para la salud humana available at https://www.gacetaoficial.gob.pa/pdfTemp/28715/GacetaNo_28715_20190215.pdf (July. 2022)